Company Tests Mobile Health for Tuberculosis Treatment
The day after emocha® Mobile Health, Inc., launched in 2013, CEO Sebastian Seiguer (pronounced SAY-ger) heard about a 3-year-old tuberculosis (TB) patient being treated at a Baltimore clinic. Maryland requires healthcare providers to watch patients take every dose of their medication for active TB treatment, an approach known as directly observed therapy (DOT). But coordinating with the clinic every day was proving too difficult for the girl's mother to manage. To keep up with the treatments, she would have had to quit her job.
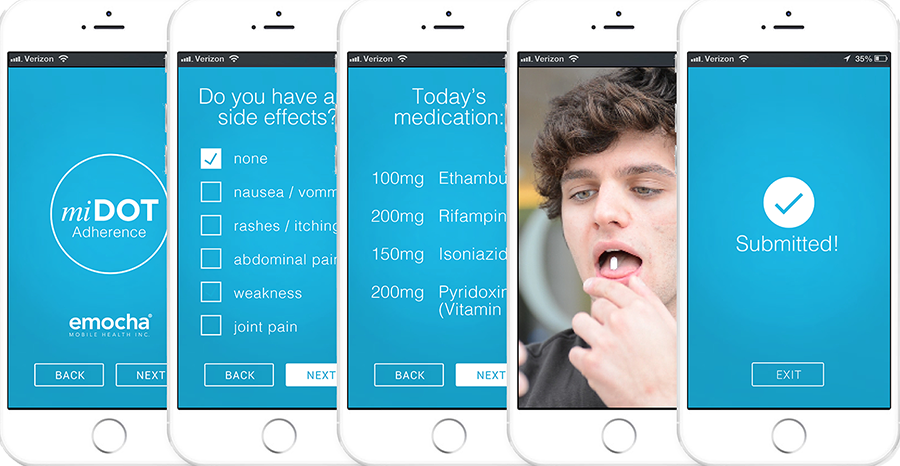
The girl's case became the first test of mobile internet DOT (miDOT®), a new technology developed by emocha to allow patients to report side effects, record video of themselves taking their medication, and securely upload the data for a healthcare provider to verify. Such smartphone-enabled healthcare applications are often classified as mobile health, or mHealth, technologies.
With the support of an NIMHD Small Business Innovation Research grant, Seiguer's team collaborated with researchers at the Johns Hopkins University Center for Clinical Global Health Education to test adherence to a daily medication regimen. Twenty-eight patients using miDOT participated at public health clinics in Baltimore and elsewhere in Maryland. The research team is testing whether miDOT can be as effective as DOT and at what cost.
“Our preliminary data show we can secure 93 percent medication adherence using video observation and can save more than $1,000 per TB patient over 6 months with software costs included,” said Seiguer.
Data gathered from the NIMHD-supported study have helped emocha expand miDOT to public health departments in Rhode Island, Seattle, and three California counties; miDOT has also been added to existing projects in Harris County, TX. A patent is pending.
Health Disparities in Tuberculosis
In 2015, U.S. public health officials reported about 9,500 new cases of TB, according to the Centers for Disease Control and Prevention (CDC). Foreign-born patients accounted for two thirds of these cases. There are notable racial and ethnic disparities: Compared with Whites, who have 0.6 cases per 100,000 people, there are 5 to 6 TB cases per 100,000 people among Hispanic or Latino, African American, and American Indian or Alaska Native populations. Pacific Islander and Asian communities have more than 18 TB cases per 100,000 people.
TB treatment aims to cure patients, prevent transmission, and prevent drug resistance. Since treatment takes several months to complete, adherence over the long term is a central focus of care. CDC treatment guidelines recommend using DOT rather than self-administered therapy.
DOT has been in place for decades, and when Seiguer and his team launched emocha, TB clinicians considered miDOT “preposterous,” he said. Many remembered expensive wall-mounted video phones from the past and thought patients would resist using mHealth technologies. They were concerned that the system would not be able to protect patients' health information adequately, and some believed miDOT would undermine the in-person relationships that providers develop with their patients. In practice, Seiguer said, those concerns have been largely refuted.
Building Value from Research Data
When they've asked patients for feedback, “One claimed miDOT saved their life because otherwise they were going to stop treatment to avoid coming into the clinic every day,” said Seiguer. “The stories from patients convey autonomy and more control over their health and their daily life.”
Seiguer and his team plan to use the data they have collected to directly support sales of the technology. In addition, the team hopes to win a Phase 2 grant to support research with more patients and to expand miDOT's use nationally, adding sites in California, Colorado, Idaho, Michigan, and Puerto Rico. They have also proposed studying the platform among patients with latent TB.
Simultaneously, emocha is eager to validate miDOT's effectiveness in monitoring medication adherence for conditions other than TB. Pilot testing in hepatitis C treatment is underway, and Seiguer expects to start testing miDOT as a solution for adherence to office-based treatment of opioid addiction in the spring.
Optimizing his team's efforts in three very different but interdependent areas—academic theory and research, engineering, and commercialization—is one of Seiguer's proudest accomplishments. “I'm so happy that we untertook such a formalized study with our collaborators at Johns Hopkins,” he said. “Now we can approach the market with compelling evidence and strong research data that communicate the efficacy of the intervention.”
“We are so fortunate to have had the opportunity to study the intricacies of medication adherence using our technology,” Seiguer continued. “It's amazing that such resources are available to start-up companies. The NIMHD support has not only made our technology better, it has laid a solid scientific foundation for our commercial roadmap.”
Posted May 23, 2017